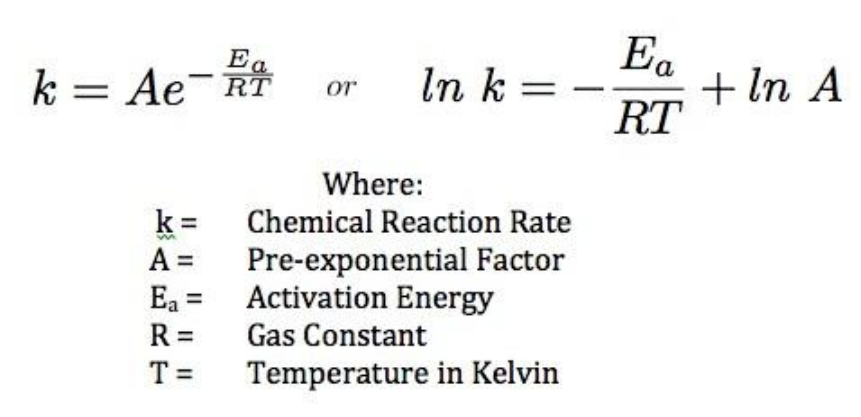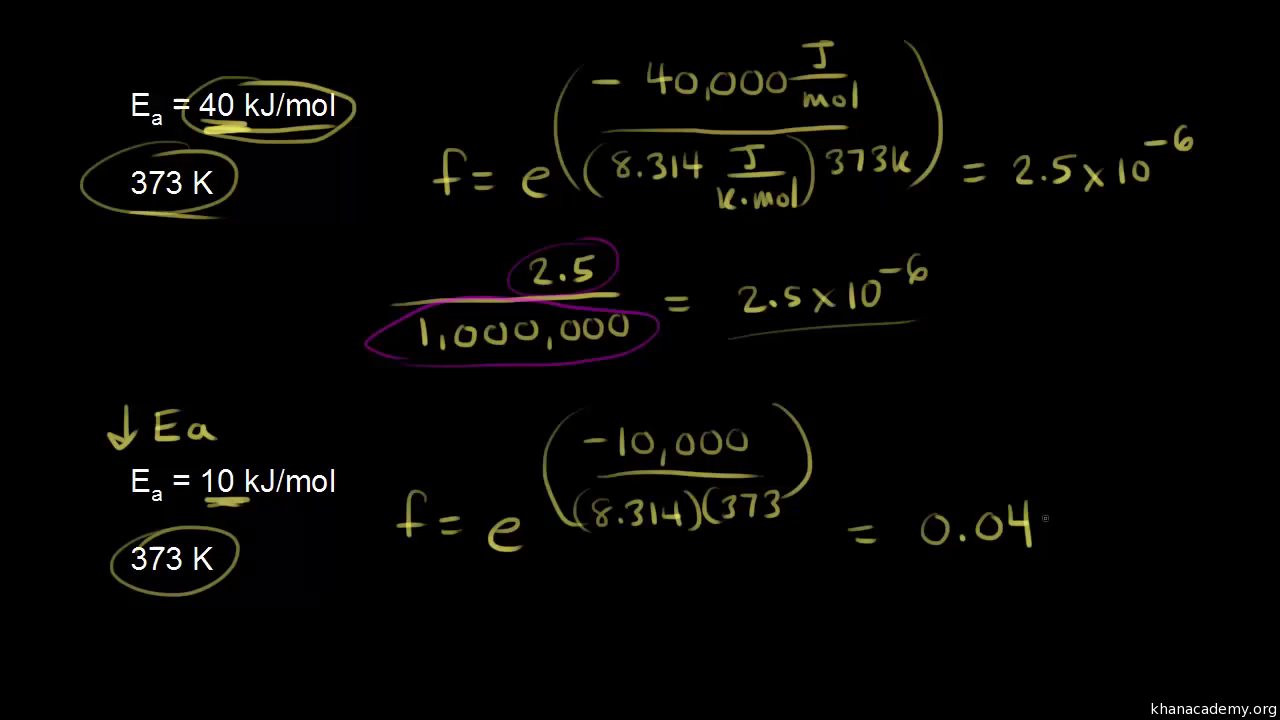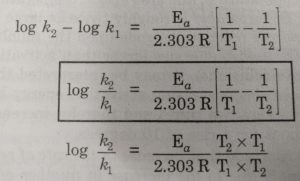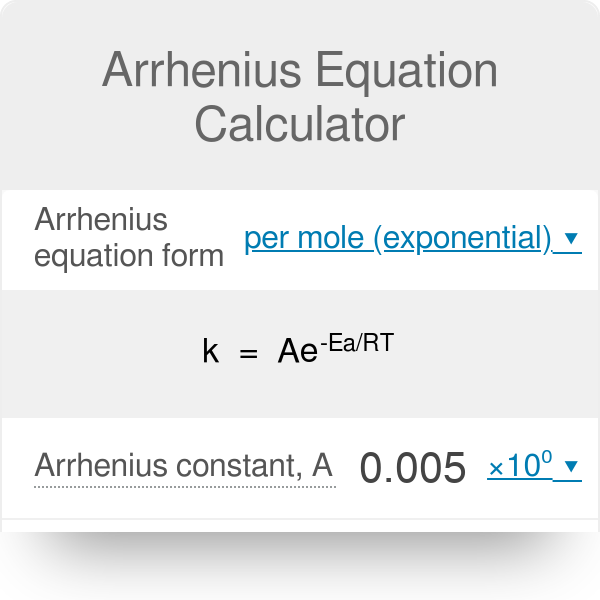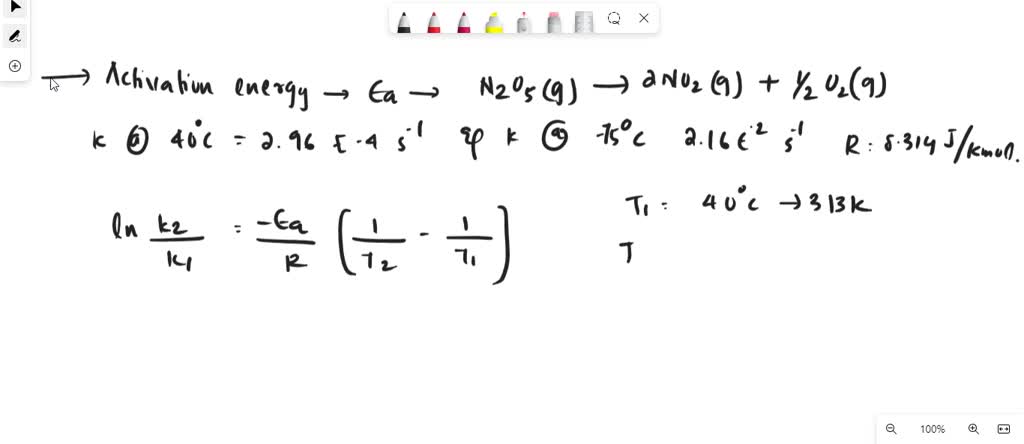
SOLVED: Calculate the activation energy, Ea, for N2O5(g) â†' 2 NO2(g) + 1/2 O2(g) given k (at 45.0 °C) = 5.79 × 10^-4 s^-1 and k (at 60.0 °C) = 3.83 ×

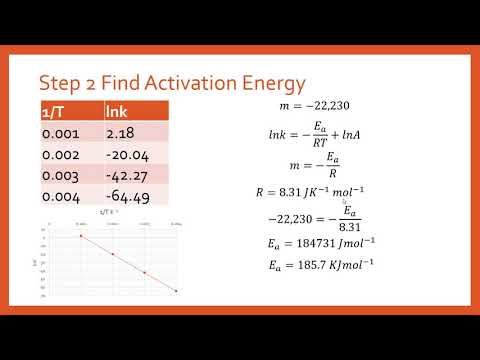
16.3.2 Determine activation energy (Ea) values from the Arrhenius equation by a graphical method. - YouTube




![Kannada] The rate constant of a reaction is doubled when the temperat Kannada] The rate constant of a reaction is doubled when the temperat](https://static.doubtnut.com/ss/web-overlay-thumb/6625533.webp)