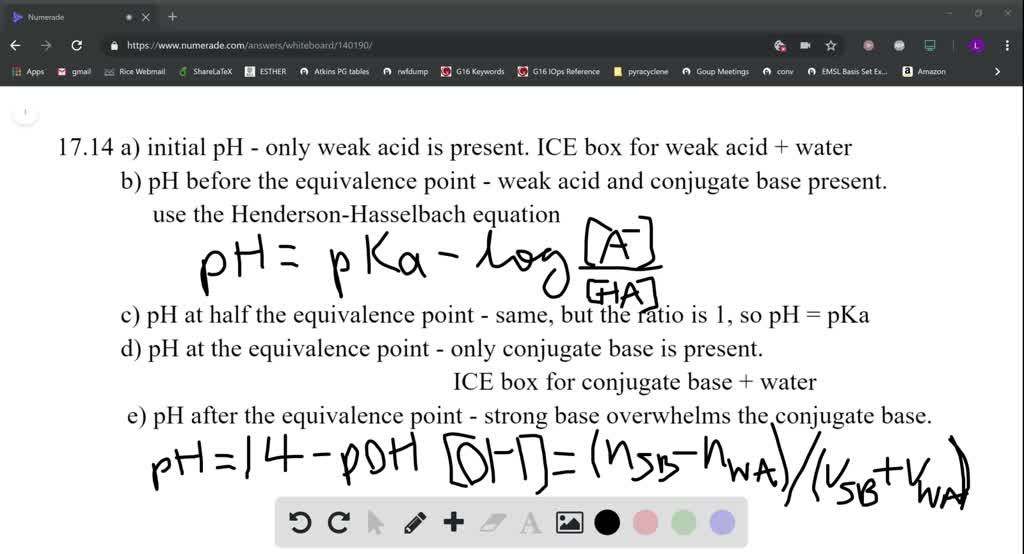
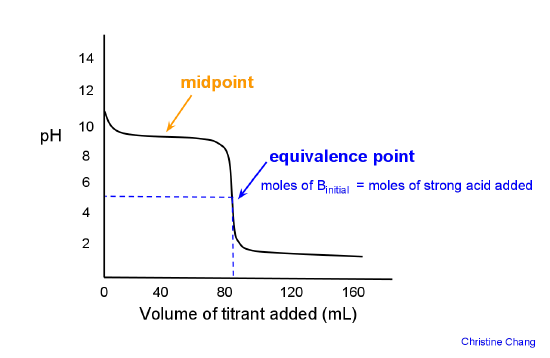
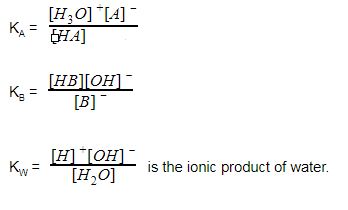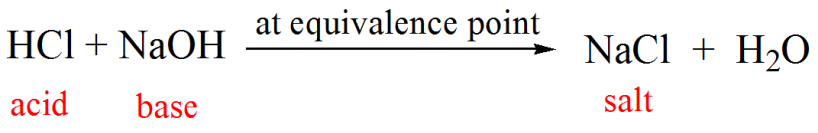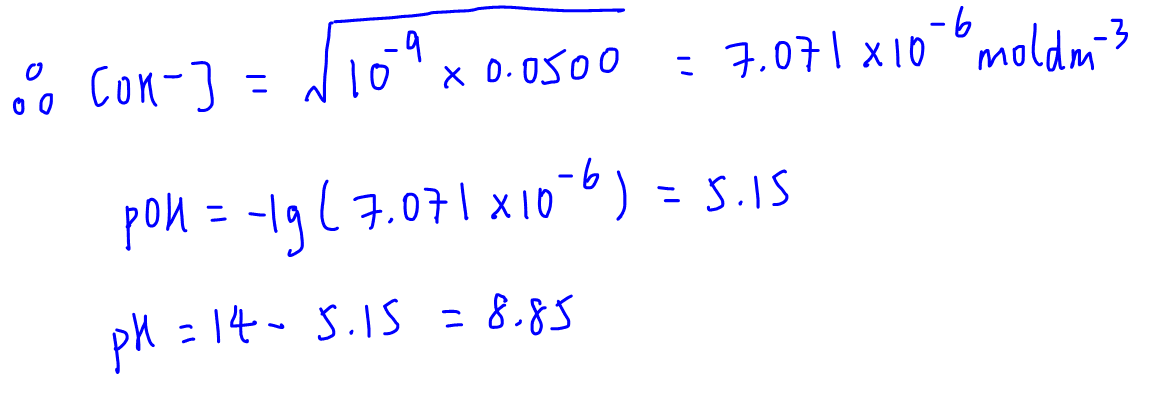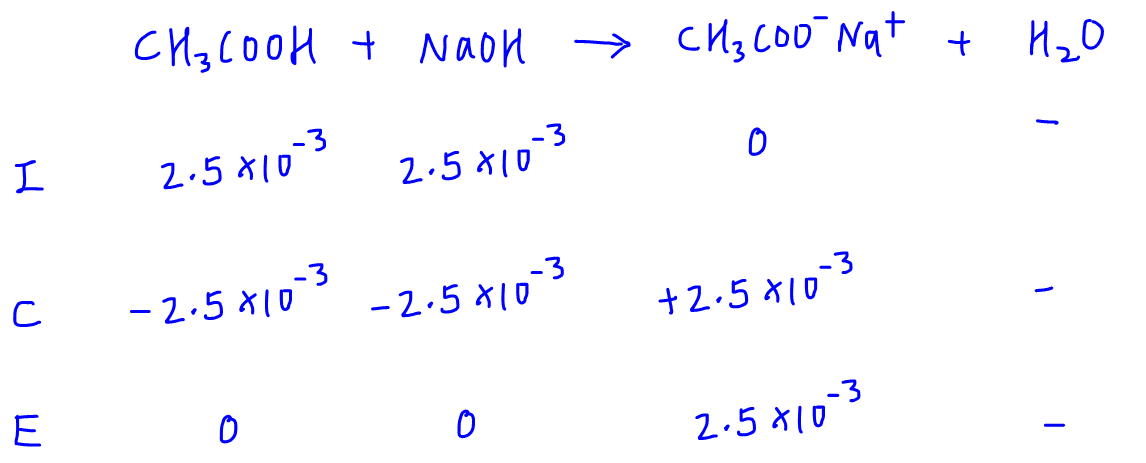How to Calculate pKa From the Half Equivalence Point in a Weak Acid-Weak Base Titration | Chemistry | Study.com

OneClass: Calculate the pH at the equivalence point for the following titration: 0.20 M HCl versus 0....

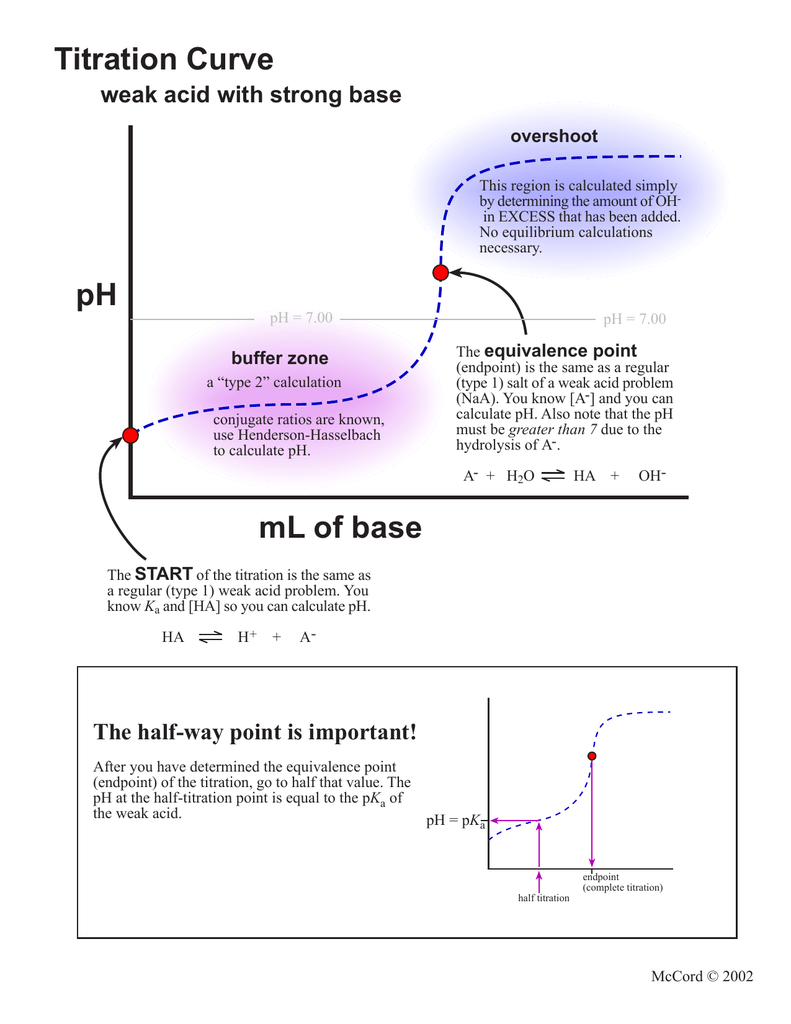
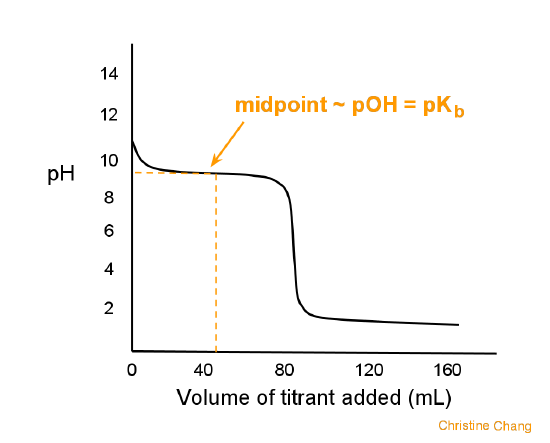
Acid/Base Titrations. Titrations Titration Curve – always calculate equivalent point first Strong Acid/Strong Base Regions that require “different” calculations. - ppt download

acid base - Titration curve graph, finding exact point of the equivalence point - Chemistry Stack Exchange
![Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)] Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/328699097_web.png)
Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]