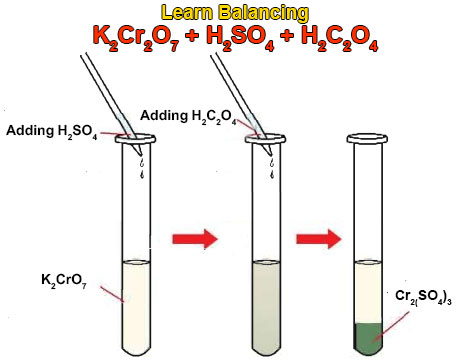
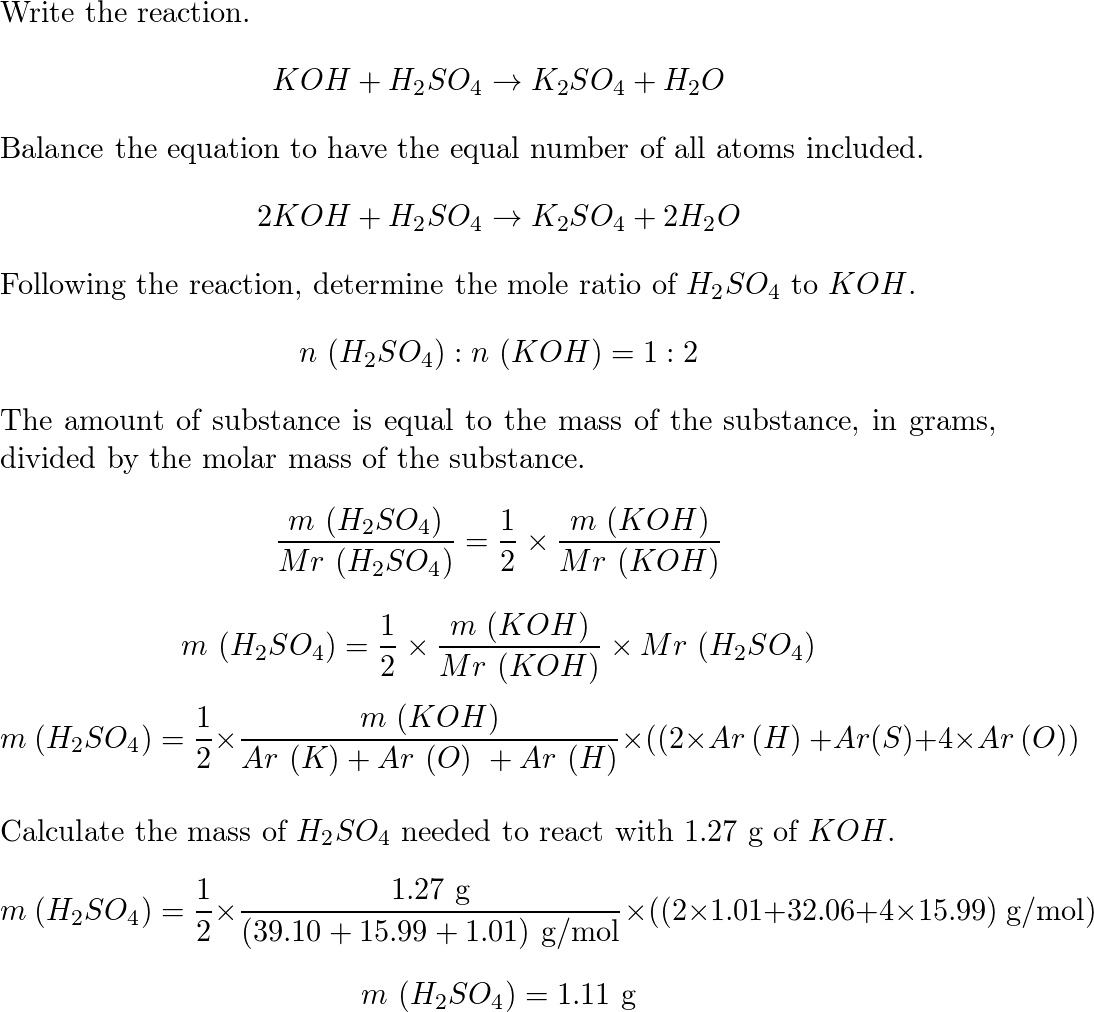Balance the following equation by oxidation number method : (i) K2Cr2O7 + KCl + H2SO4 → KHSO4 + CrO2Cl2 + H2O - Sarthaks eConnect | Largest Online Education Community

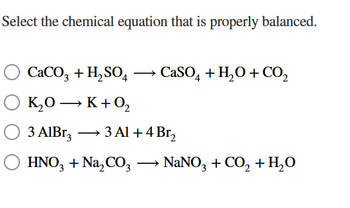
Which is the correctly balanced chemical equation for the reaction of KOH and H2SO4? A,B,C,or D? - brainly.com

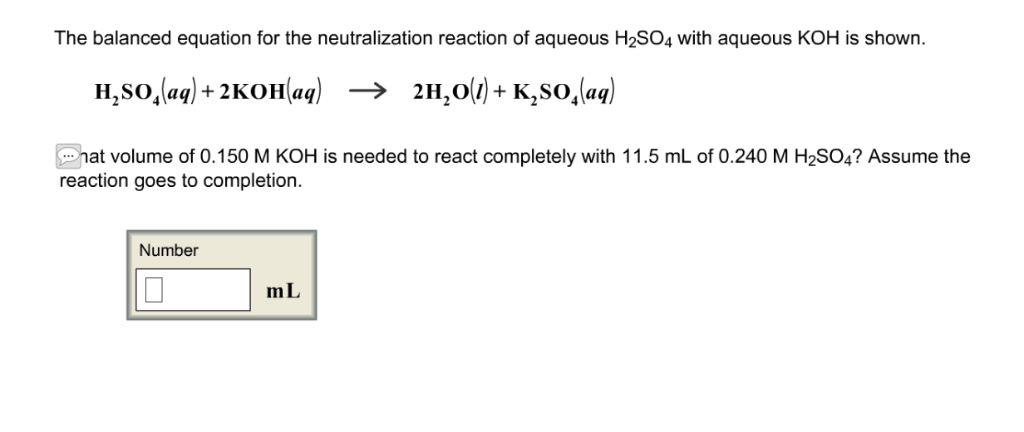
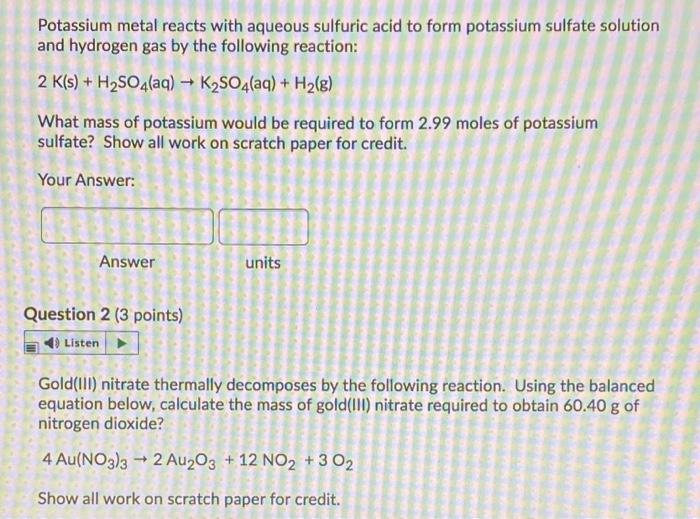
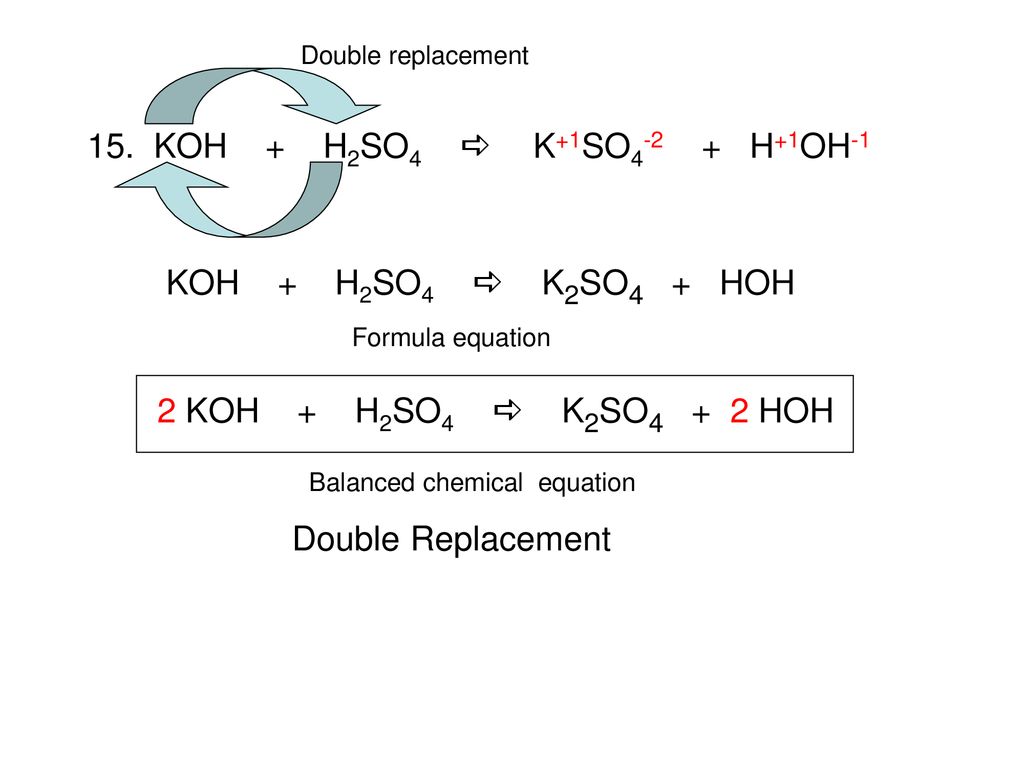
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) | Understanding, Balance, Molecules

T DIVERTI (D) Juipuur In the reaction, KI + H2SO4 -- K2SO4 + 12 + SO2 + H2O The elements undergoing oxidation and reduction respectively are : (A) K, 1 (B) S, I (C) I, S (D) SK