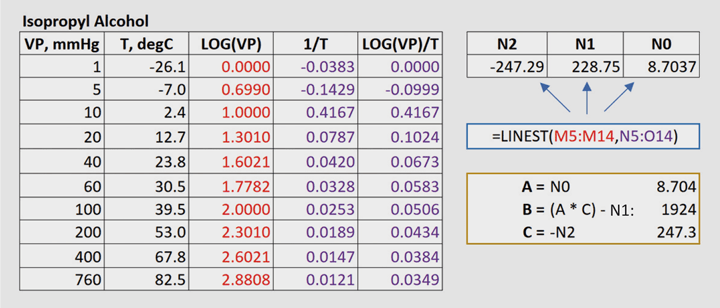
The vapour pressure of a pure liquid 25°C is 100 mm Hg. Calculate the relative lowering vapour pressure the mole fraction of solvent in solution is 0.8. pº - PS = Xsolute

a) Vapour pressure of benzene is 200 mm of Hg. When 2 gram of a non-volatile solute dissolved in 78 gram benzene. Benzene has vapour pressure of 195 mm of Hg. Calculate

The relative lowering of vapour pressure of 1% Solution of Aniline in Ether was 0.007. Calculate the molecular weight of Aniline. - India Site